Wednesday, October 18, 2023
Scientism and Long Ages. Isotopy and Isochrony dating suffer from a long litany of issues.
The Earth is not billions of years old.
by StFerdIII
(How much of a ‘science’ is geology really? When you investigate the telemetry and metrics applied to ‘aging’ artefacts the sceptical reviewer is left unimpressed.)
In a previous article radio-carbon testing as a tool for measuring ‘long ages’ was analysed, and any close examination reveals C14 dating to be crude and unscientific. Radiocarbon dating does not support long ages. Yet the ‘science’ claims that such techniques are ‘gold standards’ in chronometry and age identification. It is worth recapitulating the issues with radiocarbon dating before moving on to two other problematic areas for Long-Agers, namely that of isotopes and isochrony-dating. (The next article will apply these 3 methods to the Grand Canyon and other formations which reveals the gross inaccuracy and massively exaggerated long-age dates).
1) C-14
C-14 dating is premised on the following:
C-14 comes from the atmosphere and becomes part of the food chain.
Carbon 14 dating is only used on material that was once alive. Bones, flesh, plants, and any remains that are not entirely fossilized into rock are targets.
Once a plant or animal dies, it stops ingesting new C-14. Existing C-14 in the body continues to decay, reducing the percentage of C-14 to C-12 in the physical remains.
After pulling up a sample, and assuming no contamination occurs from the handlers, a sample’s percentage of C14-to C12 is measured, compared to the atmosphere’s percentage and the time since death is then calculated.
Some obvious problems with C-14 dating which are largely ignored
The above process is holed with assumptions and basic design flaws. C-14 dating cannot give long ages of anything.
- C-14 accuracy in dating is very limited, maybe up to 25.000 years or so, given that its half-life of only 5,730 years. It can’t age artefacts to be ‘millions of years old’.
- Long-Agers are dating samples of rocks to be millions of years old, when they contain microscopic fragments of shells, bone, graphite (wood) and other organic materials. This is impossible.
- Coal is decomposed or metamorphosed plant remains crushed under great pressure which presents another huge conundrum for uniformitarians (what caused the conditions to make coal?). Coal samples from deep mines have been dated (or assumed to date) from millions of years ago, yet every single sample contains and will contain based on its composition, C-How is this possible?
- Diamonds are very dense and not susceptible to internal contamination. Long Agers routinely ascribe ‘billions of years’ for diamond formation (another issue they cannot explain, namely, the heat, the pressure, the construction of such hard carbon-based forms). Yet every single diamond sample will contain C-14 – how can they possibly be ‘billions of years old’?
- Dragon or dinosaur tissue and bones have also been dated along with petrified wood, again from locations across the world. Every single sample contains and will contain, C-14. These artefacts cannot be millions of years old. Soft tissue and DNA (very unstable) also do not last millions of years.
- The above is observational science. You can only twist these observations into millions and billions of years in age if you follow a philosophy and religious framework which demands illimitable ages as the answer.
2) Rocks of Ages
The accepted theory about rock creation is that igneous rock is formed when it first cools down from a molten or semi-molten state, which may include a variety of elements, including radioactive ones. Within this cooling period radioactive elements decay from heavier larger atomic elements (parent) into smaller atomic elements (daughter) that are more stable.
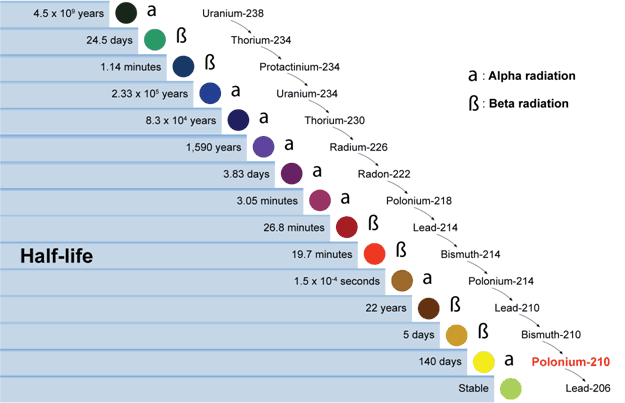
An example is uranium (U) which decays into lead (Pb), a process which was confirmed by 1900 (Marie Curie discovered Polonium, see figure below). So, for the past century and more, there is observational science on the decay rate from uranium to lead and this has allegedly been proven stable.
Due to the assumed ‘stable rate of degradation’ (rates are in the above table), the amount of parent / daughter elements present today in a rock sample, can be used to calculate backwards to the estimated age of when the rock was first formed. This method is used only on metamorphic and igneous rocks – not sedimentary rocks (which are rocks laid down by water, where fossils are primarily found). However, this technique of radio-dating using uranium to lead decay (or back up the chain from lead), is based on a series of assumptions, which greatly qualify any findings:
Assumption 1: Geology is a closed system. A similar problem exists for the Klimat Cult. The earth is not a closed system, and neither is its geology. This is a big problem. How would anyone know if there has there been contamination into the rock of either extra amounts of parent or daughter elements? What if extra lead entered the rock (hydrothermal explosion), or contamination occurred affecting lead and uranium?
Assumption 2: The decay rate has not changed. A 100-year sample of decay rates appears to be rather inadequate when talking about millions of years. No one knows if the rate of decay has changed in the past 1.000 or 1.000.000 long age years. To be honest, one should doubt the given and accepted decay rates, since finding confirmation of said rates is very difficult.
Assumption 3: There is no lead in the rock when it is first formed. This does not seem valid, given that hyrdothermally produced lead is found in all 3 rock types. To assume for example, that a rock starts only with uranium and no Pb (lead) is a gross simplification and likely incorrect. (Isochron dating, see below, which relies on multiple rock samples, is an attempt to correct this, but still has underlying assumptions based on 1 and 2 above.) More here