Saturday, October 14, 2023
$cientism and Long-Ager Theology. Radio-Carbon dating and its inconvenient issues.
As with Evolution, Big Banging or other Long-Ager Gospel, C14 dating is riven with impossibilities and contradicts common sense and observational evidence.
by StFerdIII
Carbon 14 dating is used as ‘scientific and consensus proof’ that the Earth is 4.5 billion years old and various ‘fossils’ found in our geological strate are ‘millions of years’ in age. 14C dating is probably the foundation stone for many belief systems around long ages. But a question rarely asked is, ‘how scientific is carbon-14, or 14C dating?’ Given that so much of ‘settled science’ is junk and bunk, how valid are the techniques and assumptions behind this technique which allegedly ‘proves’ Long-Ager theology?
What is 14C?
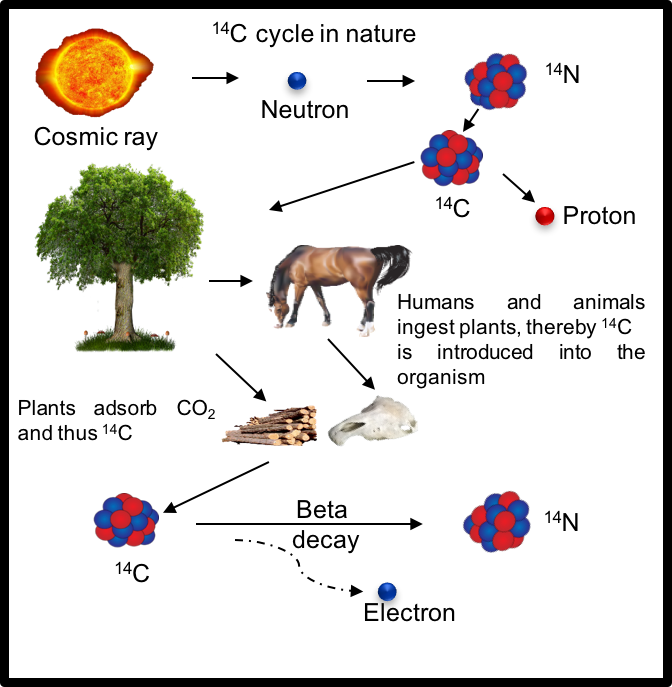
Carbon is the essential and unique element for life on Earth. Carbon is also expressed in charred wood, diamonds, and graphite in ‘lead’ pencils. Ordinary carbon (12C) is found in the carbon dioxide in the air, which is taken up by plants, which in turn are eaten by animals. So a bone, or a leaf of a tree, or even a piece of wooden furniture, contains carbon.
Carbon comes in several forms, or isotopes (another word for form). One rare form of carbon contains atoms that are 14 times as heavy as hydrogen atoms: carbon-14, or 14C, which is also called ‘radiocarbon’. When 14C has been formed, like ordinary carbon (12C), it combines with oxygen to give carbon dioxide, and so it also gets cycled through the cells of plants and animals. This known fact is the premise of the dating technique around carbon-14.
Carbon-14 is made when cosmic rays knock neutrons out of atomic nuclei in the upper atmosphere. These quick moving displaced neutrons then collide with ordinary nitrogen (14N) at lower altitudes, converting it into 14C. The most prevalent gas in our atmosphere is nitrogen and the interplay between displaced atomic nuclei and nitrogen gives rise to the element 14C. Unlike common carbon (12C), 14C is unstable and slowly decays, changing back into nitrogen and releasing energy. This instability makes it radioactive.
14C dating
The main idea behind 14C dating is to count how many 12C atoms (normal carbon) exist for every 14C atom (radiocarbon), and calculate the 14C/12C ratio. The underlying assumption is that because 14C is inter-mixed with 12C, the ratio is expected to be the same whether the sample is from flora or fauna.
In living creatures, 14C atoms are constantly changing back to 14N. However, they are still exchanging carbon with their surroundings, so the mixture remains about the same as in the atmosphere. When a plant or animal dies, the 14C atoms which decay are no longer replaced, so the amount of 14C in that once-living creature decreases as time goes on. In other words, the 14C/12C ratio gets smaller. Science calls this a ‘clock’. Obviously, 14C dating works only for things which were once living. It cannot be used to date volcanic rocks, for example.
14C and decay rates
The rate of decay of 14C is such that half of the amount will convert back to 14N in 5,730 ± 40 years. This is the ‘half-life’. So, in two half- lives, or 11,460 years, only one-quarter will be left. Thus, if the amount of 14C relative to 12C in a sample is one-quarter of that in living organisms at present, then it has a theoretical age of 11,460 years. Anything over 50,000 years old should theoretically have no detectable 14C left. That is why radiocarbon dating cannot give millions of years.
In fact, if a sample contains 14C, it is good evidence that it is not millions of years old.
Measurement of 14C in historically dated objects might enable the level of 14C in the atmosphere at that time to be estimated, and so partial calibration of the ‘clock’ is possible. Accordingly, carbon dating carefully applied to items from historical times can be useful. No one denies this.
However, even within historical calibration, archaeologists do not regard 14C dates as absolute because of frequent anomalies. They rely more on dating methods that link into historical records. In general, outside the range of recorded history, calibration of the 14C ‘clock’ is not possible and much of dating becomes a game of tautology. An example is that the artefact is found within a strata that ‘must be’ 100 MA, therefore if we date it we should calibrate the result to conform to that date. Pick your method and manipulate the outcome. More here